01/21/06
The Physical Properties of Water
Water is an almost magical chemical. Its properties never cease to amaze - imagine a chemical that gets more dense as it cools - until it reaches a point where it rapidly becomes less dense! It dissolves many inorganic materials as well as organic ones. Organisms can build macromolecules to attract or repel water as needed simply by varying the charge on side chains. Water exists primarily as a liquid at normal temperatures, yet a significant amount can often be found in the atmosphere. Its density is great enough to support the bodies of many types of creatures, often eliminating or reducing the need for elaborate skeletons. The surface tension of water helps it climb trees and support small organisms. It limits the amount of that notorious toxin oxygen that can reach an organism, yet usually carries just enough O2 to support cellular respiration. Large bodies of water heat and cool so slowly that they effectively insulate the organisms within them from daily (and sometimes seasonal) temperature changes. And, of course, water and its ability to store and move heat controls weather on our planet.
Often, in our considerations of biological systems, we, being terrestrial organisms, speak of the "problems" associated with living in water. We talk about "adaptations" to aquatic life, about the "pressures" (both real and figurative) of living in aquatic habitats. We use terms like "the cruel sea" or "the cold ocean". We are probably wrong. Life evolved in the oceans and is most at home there. Truth be known, terrestrial habitats are the most difficult and require the most "adaptations", at least in the sense that an adaptation is a modification of some original form. Still, the aquatic world is alien to us, and we will no doubt carry our prejudices and biases with us throughout this course.
The Water Molecule
Most of the physical properties of water that are of interest to biologists are the result of its unique molecular configuration. H2O is, of course, comprised of two hydrogen atoms bonded to a single oxygen atom. Water forms in an exothermic reaction whenever hydrogen and oxygen are mixed and exposed to enough energy to activate the reaction. This reaction has been known since the discovery of the two atoms, but was demonstrated most spectacularly in an experiment carried out by the German government. In 1933, the Germans filled a large envelope with hydrogen, sent it across the Atlantic Ocean to build up a large static electrical charge, and allowed the charge to equalize with a metallic structure at Lakehurst, New Jersey. The static discharge ignited the hydrogen in the envelope with atmospheric oxygen to form water. The main problem with this experiment was that it took place too close to the ground for a really large crowd to see, and, since this was before television, we had only radio reporters to relay the observations to scientists around the world. However, since there was a loss of life in the incident, some reconstructionists propose that this elegant experiment was in fact nothing more than a terrible tragedy. Since the German records were lost in the war, we may never know the truth.
Once combined, the water molecule is a strange beast. The oxygen "side" of the molecule has a slight negative charge, while the hydrogen "sides" are slightly positive (Fig. 1). This forms a bipolar molecule that can bind the oxygen side loosely to a positively charged ion or molecule, and the hydrogen side to a negatively charged ion or molecule, thus obeying Abdul's Law that opposites attract (Abdul, P. 1990, Chrysalis). This explains many of the unique properties of water. It dissolves so many different things because it can dissolve anything with an ionic charge - positive or negative. It is so dense because under normal circumstances the adjacent molecules are also water molecules and the oxygen on one water molecule binds loosely to the hydrogen on the next, and the binding pulls all the molecules together, packing them in. Surface tension is high because of the attraction of the molecules.
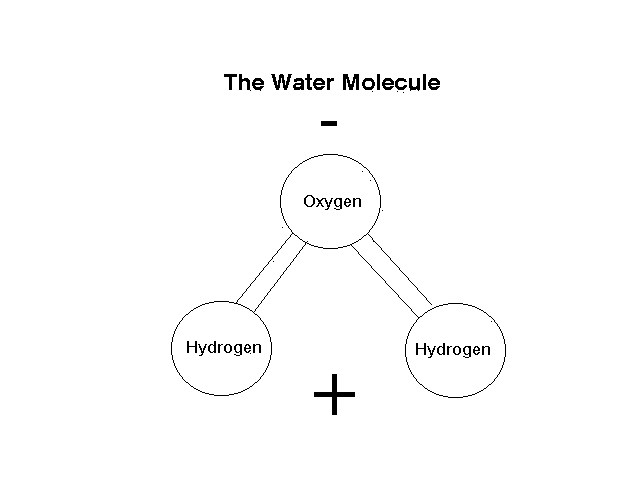
Figure 1. The water molecule, showing positive and negative polarity
Surface Tension, Hydrophilic and Hydrophobic Interactions
Water binds to hydrophilic (water-loving) surfaces because such surfaces have slight electrical charges; it beads up on hydrophobic (water-hating) surfaces because they have no charges and the water would rather mingle with itself than mess around with something that has no charge. Oil, for instance, is beautifully balanced ionically, and, you know, oil and water just don't mix. Exxon demonstrated this eloquently in 1989 with a large-scale experiment in Alaska. When some objected that sure, it works in Alaska because it's so damn cold up there, Exxon promptly repeated the experiment (though on a smaller scale) at sites all over the United States. Ironically, although oil and water don't mix, crude oil does mix with the skin oils painstakingly groomed into the fur of sea mammals and the feathers of sea birds, where the natural skin oil waterproofs the keratinaceous outgrowths and allows them to trap air, which acts as an insulator. Crude oil, however, mixes with the skin oils and mats the hair (feathers) down flat, eliminating the air space and the insulation. And, of course, the oil just tastes yummy when it sits in the belly of some poor critter that tries to lick it off so it doesn't freeze to death. Again, Exxon is currently conducting research into these effects.
Detergents destroy surface tension by insinuating their ions among the water molecules and binding them together more loosely than they would normally bind. In your washer this is good, because the detergents allow the water to get in and lift out the soils. In a stream, this is bad, because all the water striders suddenly sink.
Other organisms use "detergents" in creative ways. For instance, the whirligig beetles (Gyrinidae) secrete a surfactant - a chemical that reduces surface tension - behind them. They are then "drawn" forward by the surface tension. Whirligig beetles are unusual in several instances. They have 2 pairs of compound eyes, one pair above and one pair below the water. The also use chemicals other than surfactants. They have an aggregation pheromone that supposedly smells like Juicy FruitTm gum (the gum can be used to attract the beetles), and they smell like ripe apples (a defensive secretion). They use echolocation to find each other, avoid obstacles, and find food.
Organisms that exploit the surface tension of water are numerous. Some use the surface tension as a platform to support their weight above the water; others suspend themselves from the surface of the water. For small organisms, the surface tension of water creates a significant barrier to their movement in or out of the water. The trick in dealing with surface tension is to fashion biomolecules to interact with the water in an appropriate way. Add positive and negative ions to your molecule in the right places and you create a polar molecule which will be hydrophilic (the water would rather bind to it than to other water molecules); eliminate all ionic imbalances and you create a hydrophobic molecule which will not interact with water. Vaseline and waxes are examples of hydrophobic molecules; organisms often use waxes to make a surface hydrophobic.
The interplay of hydrophobic and hydrophilic surfaces with water can perhaps best be seen by examining mayflies. These organisms have aquatic larvae with hydrophilic surfaces. These surfaces allow the larva to exchange gasses (O2, CO2) with the surrounding water, which can come very close to the hydrophilic skin. At maturity, the larval mayfly molts to the winged subimago, the stage that must break through the water's surface. The subimago is covered with small waxy hairs and beads that are hydrophobic. The subimago floats to the surface and is literally pushed out of the water as the polar water molecules attempt to reform behind the subimago. The subimago can float comfortably on the surface of the water for several seconds - long enough to provide a target for trout, and the basis for fly-fishing - before taking off. It will fly to streamside vegetation and molt again; it is the only insect to molt once it has reached the winged stage. This second molt gets rid of the awkward, heavy, waxy coat of the subimago. The adult mayfly is quicker, but must avoid getting too close to the water. Some mayflies that do come close to the water during their later mating flights may retain some parts of the body (particularly the underside) in a hydrofuge (hydrophobic) state. See the paper by Edmunds and McCafferty (1988) for more details on this interesting transition.
On the surface, water striders (Fig. 2), Collembola, and a host of other small animals try to avoid making a transition - that is, sinking. They all have hydrophobic bodies with one exception - the claws. The claws are hydrophilic and penetrate the water surface, allowing the animal to "get a grip" on the water surface. Vogel (1988) has constructed the "Jesus Number" - an index of the practicality of an organism walking on water:
T |
= surface tension | (0.073 Newtons/meter in
freshwater,
|
p |
= density of organism | (kg m-3) |
l |
= length (perimeter) of organism in contact with water | (m) |
If Je is greater than 1, it is possible for the organism to walk on water. We won't do anything much with this equation; other than to note that the limits on an organism are primarily a function of its size, since surface tension, gravity and density are all pretty much fixed.
 Figure 2. A water
strider on the surface. The water displaced in the
"dimples" on the surface of the water weighs the same
as the insect. Hydrophobic hairs on the legs do not penetrate the
water surface; the surface tension holds the water together and
the water surface bends rather than breaks. An illustration of
the "Jesus number"!
Figure 2. A water
strider on the surface. The water displaced in the
"dimples" on the surface of the water weighs the same
as the insect. Hydrophobic hairs on the legs do not penetrate the
water surface; the surface tension holds the water together and
the water surface bends rather than breaks. An illustration of
the "Jesus number"!
Other organisms suspend themselves from the surface. Mosquito larvae, for instance, have hydrophilic bodies with a fringe of hydrophobic hairs near the breathing tubes at the posterior end of the body. The hairs can be bunched into a small tube and "punched" through the surface (or withdrawn). On the surface, they spread out and anchor the organism to the surface, and the larva is then able to breathe air from the atmosphere. One method for dealing with such larvae is to cover the surface with oil or detergents. The oil will smother the larvae since they cannot anchor in the oil layer, which is also hydrophobic. The detergent, of course, will lower the surface tension (the numerator in the number Je, above).
Density and Buoyancy
Density changes in water as it warms or cools have profound impacts on aquatic organisms. Density is also a major component of two other important parameters - dynamic and kinematic viscosity. All three of these parameters decrease (usually) with increasing water temperature. Dynamic viscosity represents the "stickiness" of water - how easily it flows (molasses has high dynamic viscosity). Kinematic viscosity is dynamic viscosity divided by density, a useful parameter in some calculations because it eliminates the effects of mass. It is easier to swim (or move blood) in warm water, but it's harder to float.
Floating in H2O is made somewhat easier due to the density of water. Most organisms have bodies that are at least 90% water, which means that at least 90% of their body weight will be offset by the weight of the water around them. It's that last 10% that is critical. Organisms that live on the bottom of a body of water are called benthic organisms. Efficient locomotion for them often entails walking or crawling on the substrate. Just as on land, in order to walk on the bottom, they need traction, and traction is largely determined by weight - or, more accurately, the downward pull on the body caused by gravity, which, as we have just seen, is largely offset by water for aquatic organisms. For instance, try walking in a pool. The shallower the water, the easier it is to walk. As depth increases, so does the effort you must put into walking. This is partly due to the increased effort it takes to move the water out of your path, but you'll also notice that in deep water, over chest height, you have a little more "bounce" in your step, and it gets hard to walk. Benthic organisms usually deal with such problems by making that last 10% of the body as dense as possible; they may have heavy shells, massive bones, or even construct cases out of rocks to carry around.
Swimming organisms (nekton), or floating organisms (plankton), have the opposite problem, and their solution is similar. They make the remaining 10% of their bodies as light as possible. Fats and oils are two biological molecules that are less dense than water and allow some organisms to float. Fats and oils are also a storage medium for energy and can be used for insulation in warm-blooded animals. Sharks have large, oil-filled livers which help them float; Flipper and other porpoises kill sharks by striking them in the liver with their snout, rupturing the liver. Trapped air is also effective as a float, of course. The Portuguese man-of-war has a gas float; waterfowl float high in the water due to air trapped in their feathers and the hollow bones in their skeletons. Fish have air-filled swim bladders (some completely isolated from the outside atmosphere and regulated through gas exchange with the blood); bladderworts (a floating plant) have air filled cavities (bladders).
Very small organisms also try to manipulate their density, but their efforts are often more difficult to explain. Diatoms, for instance, must stay near the surface to obtain enough light for photosynthesis, yet they have a heavy shell of silica. They offset the shell to some extent by a store of oil, but they still "worry" about sinking. Fortunately, the world of very small organisms is governed by different rules than the world we live in. To them, water is more like molasses and sinking rates are very small, often small enough to be offset by local currents caused by the water heating and cooling. The heating and cooling of the water also changes its density, and, for small organisms in particular, this is not a trivial matter. The change in density of water from 4o C to 25o C is large enough to mean the difference between floating and sinking in many organisms with densities near that of water. It is easier to float in denser water, and fresh water is most dense at 4o C (Fig. 3). Salt increases density of water (Fig. 4); it is easier to float in salt water as opposed to fresh water, and easier still in hypersaline environments such as the Great Salt Lake in Utah. It is not unusual for populations of small organisms to undergo seasonal morphological changes at least partially in response to temperature induced water density changes; one of the best examples is Daphnia (see Vogel, 1981, pages 23-24).
Figure 3. Relationship of water temperature to density for distilled water.
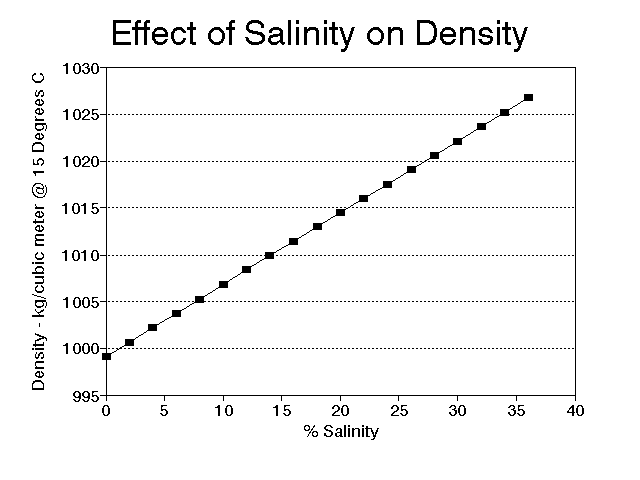
Figure 4. Relationship between percent salinity and density of water at 15o C.
The general equation governing sinking rates in water for small spherical organisms is given below:
![]()
Where U is equal to the sinking rate, a = the radius of the sphere, g is the gravitational constant (9.8 m s-2), p is the density of the organism, po is the density of the medium, and m is the dynamic viscosity (kg m-1 s-1). Note that if the density of the medium is greater than that of the organism, U will be negative - a "floating" rate.
Boiling and Freezing - Temperature and Heat
The fact that ice floats is due to the sudden reversal at 4o C of the general trend of decreasing temperature = increasing density. This is due to the ice crystals forming; the average distance between the water molecules in ice is greater than it is between water molecules at 4o C. The farther the molecules are apart, the fewer will fit in any given volume - that means less density (are you starting to appreciate density yet?). Ice formation is discouraged by molecules that get in the way of the water molecules - salt (Na+, Cl-), ethylene glycol, etc. These molecules lower the freezing temperatures of water, and allow some organisms to exist below the "freezing point" (of pure water). Furthermore, the freezing of water releases large amounts of heat. This means that water is very temperature stable at the freezing point. Winter weather is moderated to a great extent by this factor. Winter temperatures will stay near 0o C until all the local water is frozen. By the same token, it takes a lot of energy to melt ice, and local temperatures will not warm up until the ice is melted. You are probably more aware of the other end of the spectrum - when water boils. As you well know, the temperature of a pan in which water is boiling will not exceed 100o C until all the water has boiled out. This means that you can boil water in a paper cup - but watch out when the water is gone!
Boiling is the rapid evaporation of water. Unlike freezing, which does not occur until all the water is at 0o C; evaporation can occur at any temperature as long as the atmosphere is not already saturated with water. The ability of air to hold gaseous water is also correlated to temperature; the warmer the air, the more water it can hold. In the winter, a house takes outside air, which is cold, and warms it. This warmer air can hold more water, and since it is "short" of water, water will evaporate from moist areas in the house (plants, people, etc.) more quickly. Therefore, you have to water plants more often, and you are more likely to catch cold, since your respiratory linings can dry out and be unprotected from viruses. When warm air is cooled, it can no longer hold water and water comes out of the air. If the ground cools off, it will pick up H2O from the adjacent air (dew); as water vapor rises through the atmosphere it cools and the water condenses and falls to the ground (fog, clouds, rain). Moist air also holds more heat and "feels" warmer to us (although moist air and dry air at the same temperature are at the same temperature we perceive the moist air as "warmer").
The large number of molecules per unit of volume (density) of water means that the overall kinetic energy of all those molecules combined (heat) will also be large. In addition to the "extra" heat that must be added or extracted when changing the physical state of water from solid to liquid to gas (or gas to liquid to solid); it takes a lot of energy (heat) to change the overall kinetic energy of the densely packed water molecules. It is important to realize the difference between heat and temperature, temperature is a quality, heat is a quantity. Temperature measures how fast (how much kinetic energy) a group of molecules is moving; heat measures how many molecules have that energy. Water, being dense, has a lot of molecules, therefore, a lot of heat, at least compared to air. The specific formula for heat is:
H = m x t x sh
Where H = heat, m = mass (grams), t = temperature (degrees C), and sh = specific heat, a constant that varies from material to material (and also varies with temperature).
We measure heat in calories, with a calorie being defined as the heat needed to raise one gram (one ml) of H2O at 15o C one degree C. Thus, substituting 1 for H, m, and t in the equation above, we can see that the sh for water at 15o C is also equal to 1. The same amount of heat will have a different effect on other materials, or on water at different temperatures. The calorie mentioned above is different from the caloric values assigned to food; these are roughly 1,000 calories and are thus known as kilocalories or Calories. You should also be aware that the calorie does not exist in the SI; it is replaced by J kg-1 K-1, or the number of joules it takes to raise 1 kilogram one degree Kelvin (the SI system is the official system of science and is not the same as the metric system, although it is closer to the metric system than the English system is). Water has more heat than air not only because it is more dense, but also because it has a higher specific heat - that is, if you had a kilogram of air and a kilogram of water, it would still take more heat to warm the water one degree than the air.
The high specific heat of water, coupled with the amount of heat absorbed or released in changing its physical state, combined with the vast quantities of water on the planet, means that water plays a major role in climate and weather. Water is the greatest sink and conveyor of solar energy reaching the earth. For instance, heat carried by the Gulf Stream from the warm tropics keeps England and parts of Europe much warmer than they would otherwise be given their latitude. Organisms living in oceans may only encounter a 2 - 3o C temperature change over their lifetimes. Organisms living in sizable (pond-size) bodies of water do not experience diurnal temperature changes (except at the surface and edges), and only a 30o C change seasonally, spread out over a period of weeks. Terrestrial organisms may experience a 30o C temperature change daily - especially in deserts where there is little water vapor in the air to block heat transfer - more on that later.
Water and Light
Water interacts with the light that strikes it. These interactions are very complex, and extremely variable, and we will only briefly discuss them here. Pure water tends to absorb long wavelength light (reds, infrared) strongly, and allows short wavelength light to pass (blues, ultraviolet)(Figure 5, below). Any suspended or dissolved particles in the water increase absorption, particularly of UV (ultraviolet) light. Since the concentration of such suspended or dissolve materials varies greatly, few general principles exist. However, it is generally accepted that at depth, particularly in the ocean, the light has a bluish cast and underwater photography relies heavily on flash or artificial light to image the reds and oranges that otherwise appear black at depth due to the lack of those colors in the ambient light. Fish and other organisms that appear brightly colored to us when brought to the surface may actually be quite drab in their native habitat. This factor cannot be overemphasized when exploring the biological significance of color in aquatic organisms - they must not be judged in terms of their surface coloration (also, unless you know something about the color perception of the organisms in question, coloration is meaningless).
Water color itself is also a tricky subject. Pure water appears clear. Its surface may take on the appearance of the sky through reflection, or of the substrate underneath due to refraction, and the two - reflection and refraction - interact as the viewer changes angles relative to the water surface. Water may also take on the color of materials suspended in it, including algae and sediment. The lovely color of the Ohio River is not a reflection of the sky (that would make it gray), or even a refraction of the bottom (which you can't see even in water centimeters deep).
It should come as no surprise that water transmits the same colors - blues - as are vitally critical in photosynthesis; after all, photosynthesis evolved in water. Our traditional view of the two-peaked photosynthetic absorption curve, with peaks in the red and blue, is somewhat distorted by the presence of accessory pigments that increase absorption of long-wavelength light. Also, remember that light passing through natural water is also being absorbed by photosynthetic organisms, which means that there is competition for light at greater depths by photosynthetic organisms.
 |
 |
| Figure 5. Colors and water. The photo to the left was taken near the surface. With little water to penetrate, reds and yellows have not been absorbed and you can see yellows among the corals in the foreground. Objects in the distance appear bluish because yellow and red light was absorbed between those objects and the camera. In the photo to the right, the snorkeler's white t-shirt takes on a bluish cast as the red and yellow light needed to constitute white light have been absorbed. The actual depth is about 10 feet (3 meters). | |
All in all, however, the color of light in water is not as critical to photosynthetic organisms as the intensity of that light. Near the surface there may actually be too much light; at depth there may not be enough. Of crucial interest to us will be the phytoplankton, those organisms such as algae that photosynthesize, yet are at the whim of the currents as they lack the ability to swim strongly. The depth at which a photosynthetic organism's rate of photosynthesis balances its rate of respiration is known as the light compensation point (LCP); a small organism with little stored energy reserves cannot survive long if it sinks below its LCP. Note that the LCP will vary by species and that dissolved or suspended materials (including other phytoplankton) in the water column will absorb light and raise the LCP for all species - the LCP in the Caribbean is measured in tens of meters; in the Ohio River it is measured in centimeters. The cyanobacterium Plectonema woolei in turbid Lake Erie has virtually all the known accessory pigments. Grown under low-light conditions (it can grow in a refrigerator, apparently getting enough light when the door is opened occasionally) it is black in color. Its LCP in Lake Erie is often about 10 m, where visibility to the human eye is measured in cm.
Modification of the LCP of many organisms will, of course, have profound impacts on the ecosystem. One of the more sinister projections of a "nuclear winter" scenario is the decreased light penetration due to smoke and dust in the atmosphere. This would raise the LCP to near (above?) the surface of the ocean, and such effects would persist for a longer period of time than the small phytoplankters, with their tiny energy reserves, could hold out. They would die, and since they produce a major portion of the O2 in our atmosphere .....
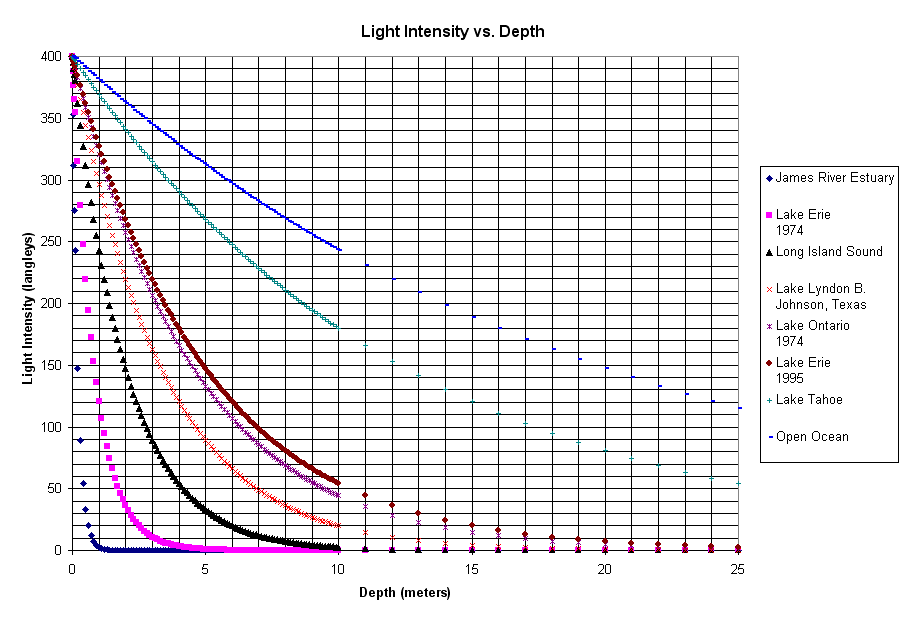
Figure 6. Light extinction vs. depth. The lines above trace the relative light intensity from the surface to a depth of 25 meters (the spreadsheet calculated the intensity every 1/10 meter down to 10 meters then every meter after that). The clearer the water (open ocean, Lake Tahoe) the less light is absorbed. Very eutrophic (enriched) habitats with a lot of plankton in the water (James River Estuary, Lake Erie in 1974) have a lot of light absorbed. Compare the line for Lake Erie in 1974 and 1995 (when it was the 3rd cleanest among these water bodies). The difference? Zebra Mussels filtering out the phytoplankton (as well as a lot of money put into sewage treatment in the US and Canada),
As light passes through water in the air, similar effects are seen. Water vapor in the air absorbs infrared (IR) strongly, while allowing UV to pass; thus, a cloud blocking the sun causes us to perceive a drop in temperature, and it is possible to get sunburned on a cloudy day. Water vapor also absorbs heat trying to leave the Earth and radiate out into space. One reason that deserts are cold at night is that there is little water vapor to block heat loss at night (the same heat loss occurs during the day, but is offset by incoming solar radiation, insolation); for the same reason, it gets cooler on clear nights than on cloudy ones. Water plays still another role in the global heat balance. Light colored objects reflect light (and heat); thus clouds, ice and snow, which are light-colored (have a high albedo), reflect heat back into space and tend to cool the planet. If you have a lot of snow, as during an ice age, this effect can feed on itself: the snow reflects the heat, which cools the planet, which allows more snow to form, which further cools the planet, and so on. The reverse takes place if things warm up, an important consideration in our examination of global warming.
Pressure
We have not mentioned pressure yet, primarily because it is of less concern to aquatic organisms than you might think. Although there are real physical/chemical effects that occur at great pressures, the biggest problems that organisms face is a difference in pressure, and most organisms deal with this very effectively. Pressure is caused by the weight of fluids (air, water), above you. At sea level, we measure pressure as one atmosphere (we'll avoid the messy SI units, pascals m-2, for now). That is the cumulative pressure of all the air. By the time we go only 10 m underwater, the pressure doubles to 2 atmospheres, and it increases by 1 atmosphere every additional 10 meters. While water is (nearly) incompressible, air is not, and air at atmospheric pressure taken underwater soon begins to compress; here is where most pressure-related problems come into play. An organism using air for buoyancy will find that air compressed as it sinks, further decreasing buoyancy and thus increasing the sinking rate, leading to a positive feedback loop. Any organs (lungs) containing air will be crushed as the air inside can no longer support them. Rising through the water column can have the opposite effect: uncontrollable ascent rates and explosion of air containing tissues. Organisms with air-containing organs are well advised to limit their vertical movement in the water column, or to enclose their air in a hard structure, such as the shell of a Nautilus. Fish caught in deep-sea trawls often explode as their swim bladders expand when they are forcibly brought to the surface. Whales, such as the sperm whale, and other organisms that routinely dive to great depths and return to the surface to breathe, have other adaptations that prevent damage (such as the lungs being small relative to the overall mass of the whale).
Humans entering water without any special equipment are limited to relatively shallow depth (30 m) for short periods of time (60 s). This was changed by the introduction of compressed air. Compressed air has enough pressure of its own to compensate for the pressure of the water, and allows the lungs to expand normally. As long as the compressed air is the same pressure as that of the surrounding water, and as long as the air can move through all air passages in the body freely (there is no congestion in the sinuses or eustachian tubes), all is well - up to a point. If the diver ascends without breathing normally, the air in the lungs is at a greater pressure than the surrounding water, and the lungs expand to the point that the thin tissues of the aleovi rupture, leading to air in the blood (and blood in the lungs), and probable death by air embolism. This effect is particularly pronounced in shallow waters, where a change in depth of a few feet without normal breathing can cause an embolism, often without accompanying pain, thus making it very dangerous. Most SCUBA (self-contained underwater breathing apparatus) classes simply involve ramming home the simple rule of breathing normally at all times, and allow the diver to become proficient enough that panic is not likely to occur and cause the diver to forget that simple rule.
From a physical standpoint, a human with compressed air could go to the deepest points of the ocean. From a physiological standpoint, however, this is not practical for several reasons. One is that oxygen is toxic, and the critical factor is the partial pressure of O2. Without going into details, this means that as depth increases, O2 becomes more toxic. To avoid this, divers going to depths below 100 m routinely use gases with less than "normal" 21% oxygen. Nitrogen, normally inert in the body, causes neurological effects (Nitrogen narcosis) at depths greater than 100 m, and is often replaced by helium (which carries body heat away so effectively that special provisions must be made to warm the diver - He also causes "Daffy Duck voice). Helium-oxygen mixtures have been tested to depths of about 600 m. Because of the costs of the specialized equipment and support teams, as well as the problem of decompression (see below), 600 m may be a practical limit for humans in the ocean. Below that depth humans need to be encased in structures (submarines, hard diving suits) that bear the pressure of the water, leaving the human at normal pressure. Recent advances in electronics have also made remotely piloted vehicles (ROV's) practical for work at great depths; witness the recent success of the ROV Jason in the discovery of the Titanic.
A further complication of diving with compressed gases is that the gasses form tiny bubbles in the blood under great pressure. As the diver ascends, these bubbles come out of solution and grow rapidly, not unlike the way a carbonated beverage bubbles when you remove the top (ever play beer hunter?). Like such beverages, agitation increases bubble formation, and the bubbles are most likely to form at the joints. The bubbles block blood flow and pinch nerves, causing the victim great pain, particularly at the joints. Victims often assume a bent, fetal position, giving rise to the common name for this syndrome - the bends. It often afflicted workers in caissons, pressurized structures built on the bottom of a body of water to facilitate construction work (bridge pylons, tunnels). A celebration of the completion of one such structure (a tunnel under the river in New York) turned out rather flat when the politicians found that the champagne wouldn't fizz under pressure. Fortunately, the local populace was treated to a joyous moment sometime later, when the politicians emerged and their otherwise boring speeches were punctuated by enormous belches fueled by great quantities of now depressurized champagne. Treatment for the bends requires repressurization in a recompression chamber, a fact known to all fans of Sea Hunt, Flipper, or Voyage to the Bottom of the Sea. Avoiding the bends requires carefully planned decompression stops on ascent, allowing the gasses to be vented slowly, a fact also well known to most fans of the above shows. Lesser known is that most sport divers are counseled to avoid decompression by planning no-decompression dives; these carefully limit the times spent at different depths to avoid build-up of N2 in the blood. A no compression dive might mean being able to stay down 1 hour at 10 meters, but only 15 minutes at 20 meters. Time spent ascending, descending, on the surface, and flying on airplanes must also be considered. The charts formerly used to calculate no-decompression (and decompression) dives are being replaced by microcomputers worn by the diver which sense depth, altitude, and time, and advise the diver accordingly. Lesser known still is that the research on which these charts are based was performed using US Navy divers during and after WWII. These men were usually about 2 m tall and had virtually no body fat, consequences of stringent recruiting practices and physical training. Recent research has shown that factors such as sex, size, and body fat can influence onset of the bends. Body fat, in particular, has an aggravating effect on the bends, and the relative safety of sport diving up until this time has been due to the extreme conservatism of the Navy scientists (who also fudged the data so that the dumber of these big guys could still use the tables in an era when an electronic calculator filled a room). The gender bias of the study was understandable given who was developing these tables, and what their motivation was; what is inexcusable was the length of time it took to replicate these studies with women (and people of different sizes and fat contents). Such data is being gathered and incorporated into such tables now.
Drag
A final physical topic related to water is the cost of moving through it. We have already seen that vertical movement - buoyancy or sinking - is easily accomplished by manipulating density and letting the forces of gravity do the rest. Horizontal movement, or vertical movement against the forces of gravity, is more complex. Two things must happen when an object moves through water. The water in its way must be moved, and the water "sticking" to it must be sloughed off. The first of these is a function of speed, size, and shape of the organism and is called pressure drag; the second is a function of speed and the amount of surface area present and is called friction drag; together they are called simply drag. As speed increases, or as size of the organism increases, friction drag becomes less important than pressure drag. This is due to the fact that friction drag is proportional to the surface area of the organism, while the amount of water that must be moved is more proportional to the volume, and, as size increases, volume increases more than surface area. Friction drag is also more important in air than in water, and at slow speeds in relation to high speeds. The type of drag that predominates can have dramatic consequences for the organism. For instance, we live in a world dominated by pressure drag, and the way to reduce pressure drag (thus increasing efficiency of locomotion) is to streamline an object, making it present a small profile to the fluid the object is moving in - a Corvette has less pressure drag than a Mack truck, even if both were the same size. However, streamlining also increases friction drag. In our world of big things and high speeds, friction drag is by far the smaller coefficient of overall drag and can be ignored, but many small organisms live under the reverse conditions and cannot afford to overlook friction drag.
How can we tell which type of drag will be most important to an organism? Fortunately, a simple mathematical relationship exists, the Reynolds number (Re):
![]()
Where p = density of the fluid; l = characteristic length of the object; U = the speed (of the object or the fluid or both); and m = the dynamic viscosity. The length is somewhat arbitrary; it is usually the length normal to the flow, i.e. the diameter of a sphere, the diameter of a cylinder normal to the flow, the length of a cylinder parallel to the flow. It is important to set up the equation so that all of the variables are in the same units - that is, you can't have a speed measured in meters per second and a length measured in millimeters. Under these conditions, Re is a dimensionless index (try it, all the dimensions cancel out). If Re<1, then friction drag predominates and flow is smooth or laminar. Between 1 and 1000, there is a transitional zone, where both factors are important but hard to predict (unfortunately, most organisms spend at least part of their lives in this zone), and flow may become turbulent (turbulence is a major factor in pressure drag). Above 1,000, pressure drag predominates, and you would expect to see streamlined structures (which work, in part, by avoiding turbulence which predominates at high Re).
Note the effect of changing any variable. Water is more dense than air, it leads to a bigger Re (the effect of the dynamic viscosity is swamped by the larger values for density); longer organisms will have a larger Re; larger Re's will predominate at higher speeds. Life at low Re is a strange world; recent experiments to simulate low Re conditions for humans used lime Jell-O (high viscosity) and have been perverted into a strange ritual staged as "entertainment" where opponents attempt to find optimum movement strategies for the low Re and best their opponents in physical contests. Apparently, wearing a minimum of clothing has been found to be adaptive under such conditions, perhaps clothing increases friction.
The Reynolds number has far-reaching effects, and we will discuss many of them this semester. One we have already discussed is sinking rates:
![]()
Once the sinking rate is calculated, it is necessary to use the value of U to calculate a Re for the spherical organism. If the Re > 1, the calculated sinking rate may not be accurate, since the equation for sinking rate assumes the laminar conditions of low Re. If the Re is greater than 1, turbulence may develop, increasing pressure drag and reducing the sinking rate. There are formulas for the sinking rate of larger (or faster sinking) objects, but we won't go into them here.
Further Reading - Physics
McShaffrey, D. and W.P. McCafferty. 1987. The behavior and form of Psephenus herricki (DeKay) (Coleoptera: Psephenidae) in relation to water flow. Freshwater Biology. 18:319-324.
- Cole, G.A. 1983. Textbook of Limnology. 3rd. Ed. Waveland Press, Prospect Hts., IL, 401pp.Read Chapters 8-9
- Edmunds, G.F. and W.P. McCafferty. 1988. The mayfly subimago. Annual Review of Entomology 33:509-529.
- Gleick J. 1987. Chaos: making a new science. Viking Penguin Inc. New York. 352 pp.
- McCafferty, W.P. 1981. Aquatic Entomology Science Books Intl., Boston. 448 pp.
- Vogel, S. 1981. Life in Moving Fluids Princeton University Press, Princeton. 352 pp.
- Vogel, S. 1988. Life's Devices Princeton University Press, Princeton. 367 pp.
